Thermodynamics is not Fundamental Physics
A lot is made of the three laws of thermodynamics in popular culture, and indeed in arguments in favour of creation. It’s easy to be fooled into thinking that these laws are cornerstones of physics, absolute and immutable. But although thermodynamics may have been the cutting edge of fundamental physics in the 1800’s, it’s nowhere near that in 2018.
Thermodynamics is comparable in this way to Newtonian mechanics. Newtonian mechanics is an excellent approximation for most practical everyday calculations, as is thermodynamics. But just as we should develop our beliefs about the world on relativity not Newtonian mechanics, we must not develop our beliefs on thermodynamics.
Statistical Mechanics
Thermodynamics was superseded by a more fundamental theory way back in the late 1800’s. Statistical mechanics explains the laws of thermodynamics by considering the mathematics and mechanics of a large collection of tiny particles.

The difference between thermodynamics and statistical mechanics can be elucidated using terminology introduced by Einstein. Einstein thought that we could distinguish two types of theories in physics: principle theories and constructive theories.
A principle theory is a theory based on broad, phenomenological statements. The starting point of the theory is its laws. Thermodynamics is an example of a principle theory, it’s based on three wordy laws:
- Energy cannot be created or destroyed in an isolated system
- The entropy of any isolated system can never decrease
- The entropy of a system approaches a constant value as the temperature approaches absolute zero
Special relativity is also an example of a principle theory, it has two postulates: the light postulate and the relativity postulate.
Constructive theories on the other hand do not use laws as their starting point, they derive them from the basic properties of fundamental entities like particles or geometry. Two examples of constructive theories are statistical mechanics and general relativity. Statistical mechanics is formulated from the basic properties of particles, general relativity from geometry and spacetime.
Statistical mechanics is the constructive theory to thermodynamics. Statistical mechanics shows us why the laws of thermodynamics come from, all three laws can be shown to be (at least approximately) true using statistical mechanics.
For Einstein, constructive theories were the aim, the superior kind of theory. The principle theories are what we deal with in the meantime, they help us move forward but we intend to replace them with constructive theories as soon as we can.
The Laws of Thermodynamics are Approximate
Although statistical mechanics can reproduce the laws of thermodynamics, the second law, for example, can only be reproduced in an approximate sense. Statistical mechanics shows us that the entropy of a system is always overwhelmingly likely to increase as opposed to decrease, but there is nothing impossible about the entropy of an isolated system spontaneously decreasing.
The second law is not the immutable statement we thought it was. Technically in fact the second law of thermodynamics is not true at all, entropy of an isolated system can decrease.
What statistical mechanics does tell us though is that although the second law is technically wrong, we can see where the law came from and that it reflects an aspect of the truth.
The Status of Energy Conservation
Now what about the statement that energy cannot be created or destroyed? Is this only an approximate law? Well, thermodynamics was principally concerned with things like heat energy and work and, at the time, it was not clear that the sum of all these kinds of energies would be a conserved quantity.
What statistical mechanics does is show that heat and work are basically just kinds of kinetic energy of large ensembles of particles. So statistical mechanics does illuminate why the first law is true, but conservation of kinetic energy is built in as an assumption in statistical mechanics – it’s an assumption about the basic properties of particles.
So really statistical mechanics is not a constructive theory with respect to energy conservation, it’s an unquestioned assumption. To know whether energy conservation is true in an absolute sense and not just in a probabilistic sense we need a more fundamental theory of physics.
Depending on how you interpret the lines on a Feynman diagram in QFT, one can see a way that energy conservation may only be an approximate law. If you take the lines on a Feynman diagram to represent virtual particles that really do pop in and out of existence then energy conservation is violated.
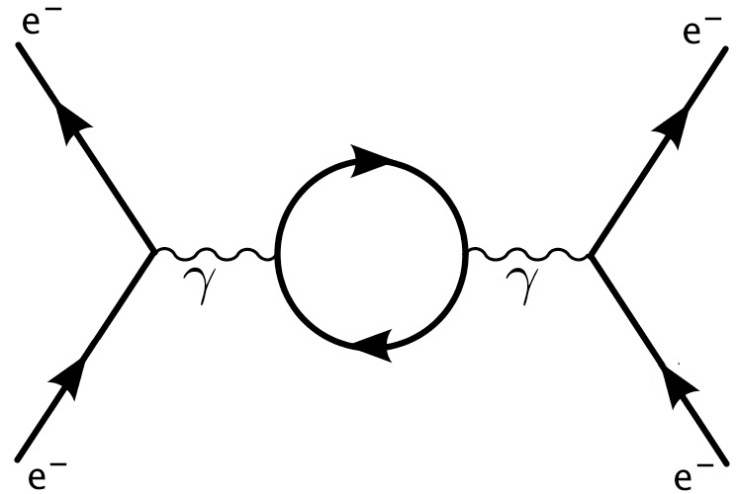
This can be seen more clearly perhaps by considering the time-energy formulation of the Heisenberg uncertainty principle. Energy can appear out of nothing, but the more energy that does appear out of nothing, the faster it has to disappear again by the uncertainty relation.
This means that energy conservation can be violated, but on a large, everyday scale this effect is far too small to notice and energy conservation is an excellent approximation.
So there are definite hints in our modern fundamental physics that energy conservation is only an approximate law, but nothing quite as conclusive as statistical mechanic’s result that entropy can decrease in an isolated system.
All in all, I really wish people would stop planting the laws of thermodynamics in their arguments as if they’re an irrefutable line of defence. Thermodynamics is 19th century physics, we’ve moved on.

Statistical mechanics still implies that the second law of thermodynamics is extremely unlikely to be violated in systems with many degrees of freedom, so the 2nd law can still be used validly in arguments that the universe probably has a beginning, or that the initial state of the universe is finely-tuned. (I assume these are the kind of arguments you are taking aim at, based on your last couple of posts.)
In fact, these kinds of arguments are just used in natural theology: they get used in physics, too. But sure, go ahead and tell Sir Roger Penrose that his qualms about inflationary theory are just based on quaint, 19th century physics. 🙂
As far as virtual particles in Feynman diagrams and the supposed energy-time uncertainty relations go, what do you think of https://profmattstrassler.com/articles-and-posts/particle-physics-basics/virtual-particles-what-are-they/ (about virtual particles) and https://arxiv.org/abs/quant-ph/0609163 (about the energy-time uncertainty relation)?
LikeLiked by 2 people
I think that the bigger issue isn’t that people cite thermodynamics so much as it is that people often cite it completely incorrectly. I can’t tell you how many people I’ve come across who mistakenly think that the second law of thermodynamics is “order cannot come from chaos.”
Explaining that it says no such thing is time consuming and annoyingly frequent.
LikeLiked by 1 person
Totally agree, it just shows a complete lack of understanding of fairly basic physics.
LikeLiked by 1 person